Chemical properties. Glycine and its sodium salt (E640) Chemical properties of amino acids
Which performs important biological functions in living organisms, participates in protein biosynthesis, is responsible for the normal activity of the nervous system and regulates metabolic processes. Aminoacetic acid, derived artificially, is used in pharmaceuticals, medicine and the food industry.
The food additive E640 combines under one labeling number aminoacetic acid (glycine) and its sodium salt - compounds that are used to optimize the taste and aroma of products. The supplement is safe and officially approved in most countries of the world.
Glycine and its sodium salt: general information
Glycine, also known as aminoacetic or aminoethanoic acid, belongs to a number of non-essential amino acids - the simplest organic structures that are part of proteins and their compounds. The substance, obtained artificially, is a colorless, odorless powder and has a sweetish taste.
On an industrial scale, glycine is produced by combining chloroacetic acid and ammonia. Aminoacetic acid, in turn, has the property of forming complex salts (glycinates) with metal ions.
Sodium glycinate is a salt of sodium and aminoacetic acid, which is also a substance of synthetic origin. Despite the fact that glycine and its salt are different chemical compounds, in the food industry they perform identical functions as taste and aroma modifiers, are combined under one label number and are considered as additive E640.
| Name | Glycine |
|---|---|
| Synonyms | Aminoacetic (aminoethanoic) acid, glycocol (obsolete) |
| Group | Nonessential amino acids |
| Chemical formula | NH 2 – CH 2 – COOH |
| Structure | Fine monoclinic crystals (crystalline powder) |
| Color | White (colorless) |
| Smell | Absent |
| Taste | Sweet |
| Solubility | Completely soluble in, partially soluble in. Does not dissolve in ether |
| Additive code | E640 (including sodium salt) |
| Origin | Synthetic |
| Toxicity | Safe when consumed within normal limits |
| Areas of use | Food industry, pharmaceuticals, medicine, cosmetology |
Biological role of glycine and its sources
Glycine is found in protein molecules much more often than other amino acids and performs important biological functions. In the human body, this amino acid is synthesized by transamination (reversible transfer of the amino group) of glyoxylate or enzymatic cleavage of choline and serine.
Aminoacetic acid is a precursor to porphyrins and purines, the biosynthesis of which occurs in living cells, but the biological role of this compound is not limited to these functions. Glycine is also a neurotransmitter that is involved in the transmission of nerve impulses, regulates the production of other amino acids and has an “inhibitory” effect on neurons and motor neurons.
The body of a healthy person independently synthesizes amino acids in the required quantities, so there is, as a rule, no need for their use in medicines and dietary supplements. Food sources of aminoacetic acid include animal products (beef liver), nuts and some fruits.
The effect of glycine and its sodium salt on the human body
Aminoacetic acid as a neurotransmitter performs regulatory functions and primarily affects the central and peripheral nervous system. Glycine has nootropic properties, normalizes metabolism, activates the protective functions of the central nervous system and has a mild calming effect.
Positive effects of glycine on the human body:
- reduction of emotional tension, anxiety, stress, aggressiveness;
- improved mood and normalization of sleep;
- relaxation of muscles and relief of cramps;
- increased performance;
- reducing the side effects of taking psychotropic drugs;
- reducing the severity of vegetative-vascular disorders;
- reducing cravings for alcohol and sweets.
As part of the E640 supplement, glycine and its salt do not have the above properties and have neither positive nor negative effects on the human body when consumed within normal limits. The food additive does not pose a threat to health, but in case of individual intolerance it can provoke an allergic reaction.
Potential dangers may come from impurities in the additive and low-quality food products, in the manufacture of which taste and aroma optimizers are used.
Application of glycine and its sodium salt
The applications of glycine and sodium glycinate are mainly limited to the food industry, medicine and pharmaceuticals. However, aminoacetic acid has also found application in the cosmetics industry due to its hypoallergenicity and antioxidant effect.
Cosmetics containing the E640 additive:
- medicated shampoos for weakened hair and anti-baldness products;
- anti-aging cosmetics, moisturizing creams and masks for all skin types;
- cleansing serums and toners;
- lipsticks and balms.
Crushed glycine tablets can be used to make homemade skin care products and added to moisturizing masks and creams. Aminoacetic acid promotes the penetration of valuable nutritional components into the deep layers of the dermis and enhances the effect of medicinal cosmetics.
Additive E640 in the food industry
 Glycine and sodium glycinate are actively used in technological processes for the production of alcoholic beverages. The E640 additive, in particular, is included in elite vodka, which helps neutralize the unpleasant odor and soften the harsh taste. There is also an opinion that the presence of glycine in alcoholic beverages helps reduce the toxic effect of alcohol on the nervous system and prevents hangovers.
Glycine and sodium glycinate are actively used in technological processes for the production of alcoholic beverages. The E640 additive, in particular, is included in elite vodka, which helps neutralize the unpleasant odor and soften the harsh taste. There is also an opinion that the presence of glycine in alcoholic beverages helps reduce the toxic effect of alcohol on the nervous system and prevents hangovers.
Food products that contain the E640 additive:
- strong alcoholic drinks;
- jams, preserves, jellies, ;
- packaged juices with pulp;
- enriched cooking;
- sports fortified drinks;
- sauces, seasonings and spices.
Aminoacetic acid is used not only to optimize taste and transport of biologically active substances, but also as an antibacterial agent. In particular, it is used to treat meat, fish and seafood to neutralize dangerous E. coli.
Medical use
Glycine is actively used for the treatment and prevention of diseases associated with the central and peripheral nervous system. This substance is part of pharmaceutical preparations with nootropic, sedative, anticonvulsant and hypnotic effects, and has a mild antidepressant and tranquilizing effect.
Medical indications for the use of aminoacetic acid as a medicine:
- decreased mental performance, sleep and memory disorders;
- emotional tension, stressful situations, neuroses;
- emotional instability and increased excitability;
- consequences of ischemic stroke, traumatic brain injury and neuroinfections;
- vegetative-vascular dystonia, ischemia;
- increased muscle tone, muscle cramps;
- alcohol and drug addiction, toxic effects of drugs that depress the central nervous system.
It has been proven that consuming 3 g of glycine per day has a positive effect on mental abilities and the general emotional state of a person, relieves drowsiness during the day and normalizes night sleep. The drug is also prescribed to pregnant women to reduce anxiety, children and adolescents who experience difficulties with social adaptation and concentration.
Additive E640 and legislation
The taste and smell optimizer E640 is used in food production in most countries of the world, but there is no information about the additive in the Codex Alimentarius. There have been no cases of glycine and sodium glycinate poisoning when consumed as food, so the E640 modifier is considered safe.
The additive is included in the list of officially approved products for use in the food industry in the European Union, USA and Canada. The legislation of the Russian Federation and Belarus also allows the presence of E640 in products within the permissible limits established by SanPiN. There is no data on the use of E640 as a flavor enhancer and flavoring agent in Ukraine.
Despite the fact that glycine and its salt do not have a toxic effect on the human body and are approved for use, products containing E640 can hardly be called useful. Most flavorings and aromas are used to attract the buyer's attention to low-quality products, the use of which should be minimized.
Proteins are polypeptides of a fairly large molecular weight that have a certain spatial structure, which depends on the sequence of amino acids included in the protein chain. This structure can be compact (globular proteins) or elongated (fibrillar proteins). All enzymes are globular proteins, fibrillar proteins include collagen, keratin - proteins of the skin and hair, very strong and highly extensible.
Based on their composition, proteins are divided into simple, consisting only of amino acids, and complex - complexes or covalent compounds of polypeptides with molecules of other classes:
nucleic acids – nucleoproteins;
polysaccharides – glycoproteins;
lipids – lipoproteins;
pigments – chromoproteins;
phosphoric acid residues - phosphoproteins.
The protein part of a complex protein is called apobelcom, non-protein – prosthetic group. Almost all simple and complex globular proteins are enzymes - biological catalysts of biochemical reactions in living organisms.
Tasks
Task 1. What volume of gas (under normal conditions) will be released when 0.001 mol of amino acid reacts with nitrous acid: a) leucine; b) lysine; c) proline?
Solution
Let us write down the equations for the reactions of the listed amino acids with nitrous acid. In the case of leucine and lysine, deamination occurs according to Van Slyke, and for lysine - on two amino groups contained in the molecule. Proline does not have a primary amino group, so the process stops at the N-nitrosation stage.
In reaction (a), the number of moles of leucine n(Leu) is equal to the number of moles of nitrogen liberated n(N2), so 0.001 mol, or 22.4 ml of nitrogen, is liberated. In reaction (b) a double amount of gas 2n(N 2) is formed compared to n(lys), since the original amino acid has two primary amino groups; the volume of nitrogen released will be 44.8 ml. In reaction (c), nitrogen is not released: proline is the only one of the 20 essential amino acids that has a secondary nitrogen atom in the a-position. When it is nitrosated, an N-nitroso (rather than diazo) compound is formed.
Answer: a) 22.4 ml N 2; b) 44.8 ml N 2; c) there is no release of N 2.
Task 2. As a result of treating a solution containing 9.36 mg of an unknown amino acid with an excess of nitrous acid at 748 mm Hg. and 20 °C, 2.01 ml of nitrogen was obtained. What is the minimum molar mass of this amino acid?
Solution
Reaction of amino acid with nitrous acid:

Using the combined gas law PV/T = const, let's calculate the volume of nitrogen under normal conditions (at a temperature of 0 ° C, or 273 ° K, and a pressure of 760 mm Hg): V 0 (N 2) (n.s.) = (P 1 · V 1 · T 0)/(P 0 ·T 1) = (748·2.01·273)/(760·293) = 1.84 ml.
Since the number of moles of the amino acid substance and the evolved gas N2 are the same, we will make the proportion:
9.36 mg of amino acid gives 1.84 ml of N 2;
M (molecular weight of amino acid) will give = 22.4 N 2.
M = (9.36·22.4)/1.84 = 114.
It could be valine: 
Task 3. Animal hemoglobin contains 0.335% iron (by weight). What is the minimum molar mass of this protein? How many iron atoms are included in the molecule if the molecular weight of the protein, determined by measuring osmotic pressure, is 67,000?
Solution
Mass fraction of iron in hemoglobin: C%(Fe) = /M(protein), where n is the number of Fe atoms in the protein molecule. Let n = 1, then M(protein)1 = M(Fe) 100%/C%(Fe) = 56 100/0.335 = 16700. Since the true value (by condition) of M(protein) source. = 67000, n = M source/M 1 = 67000/16700 = 4.
Task 4. During alkaline hydrolysis of 48 g of a dipeptide consisting of residues of the same amino acid, only one substance was formed - the sodium salt of the amino acid. The mass of this salt is 66.6 g. Establish the structure of the dipeptide.
Solution
Reaction equation for alkaline hydrolysis of dipeptide:
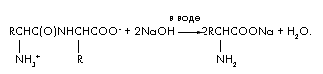
Let us denote the amount of dipeptide in moles (dipeptide) = x. Then the hydrolysis reaction consumes twice the amount of alkali (NaOH) = 2x and produces (amino acids) = 2x and (H2O) = x. Let's create a material balance equation using the formula m = ·M;
m(dipeptide) + m(NaOH) = m(amino acid salts) + m(H 2 O); 48 + 2x 40 = 66.6 + x 18; 62x = 18.6; x = 0.3 mol. M(dipeptide) = m/ = 48/0.3 = 160. M(amino acids) = [M(dipeptide) + M(H 2 O)]/2 = 178/2 = 89 g/mol. The amino acid is alanine. Dipeptide structure: ![]() .
.
Glycine (aminoacetic acid, glycocol, Gly, G)
H2NCH2COOH
Molecular mass 75.07; colorless crystals; t melting 232-236°C (with decomposition); highly soluble in water, insoluble in most organic solvents. At 25°C p K a 2.34 (COOH) and 9.6 (NH 2); R I 5,97.
In terms of chemical properties, glycine is a typical aliphatic α-amino acid. Quantitative determination is based on the formation of colored products with o-phthalaldehyde (Zimmermann reaction). It is found more often in proteins than other amino acids. Serves as a precursor in the biosynthesis of porphyrin compounds and purine bases. Glycine is a coded amino acid, non-essential; its biosynthesis is carried out by transamination of glyoxylic acid, enzymatic cleavage of serine and threonine. Lycine is synthesized from chloroacetic acid and NH 3 . In the NMR spectrum in D 2 O, the chemical shift of the protons of the CH 2 group is 3.55 ppm. The internal salt of glycine (CH 3) 3 + NCH 2 COO is called betaine.
Glycine is used for the synthesis of peptides, as a component of buffer solutions, in a mixture with other amino acids - for parenteral nutrition.
Chromatograms of samples containing this substance
Properties of amino acids can be divided into two groups: chemical and physical.
Chemical properties of amino acids
Depending on the compounds, amino acids can exhibit different properties.
Amino acid interactions:
Amino acids, as amphoteric compounds, form salts with both acids and alkalis.
As carboxylic acids, amino acids form functional derivatives: salts, esters, amides.
Interaction and properties of amino acids with reasons:
Salts are formed:
NH 2 -CH 2 -COOH + NaOH NH 2 -CH 2 -COONa + H2O
Sodium salt + 2-aminoacetic acid Sodium salt of aminoacetic acid (glycine) + water
Interaction with alcohols:
Amino acids can react with alcohols in the presence of hydrogen chloride gas, turning into ester. Amino acid esters do not have a bipolar structure and are volatile compounds.
NH 2 -CH 2 -COOH + CH 3 OH NH 2 -CH 2 -COOCH 3 + H 2 O.
Methyl ester / 2-aminoacetic acid /
Interaction ammonia:
Amides are formed:
NH 2 -CH(R)-COOH + H-NH 2 = NH 2 -CH(R)-CONH 2 + H 2 O
Interaction of amino acids with strong acids:
We get salts:
HOOC-CH 2 -NH 2 + HCl → Cl (or HOOC-CH 2 -NH 2 *HCl)
These are the basic chemical properties of amino acids.
Physical properties of amino acids
Let us list the physical properties of amino acids:
- Colorless
- Have a crystalline form
- Most amino acids have a sweet taste, but depending on the radical (R), they can be bitter or tasteless
- Easily soluble in water, but poorly soluble in many organic solvents
- Amino acids have the property of optical activity
- Melts with decomposition at temperatures above 200°C
- Non-volatile
- Aqueous solutions of amino acids in acidic and alkaline environments conduct electric current

Amino acids contain amino and carboxyl groups and exhibit all the properties characteristic of compounds with such functional groups. When writing amino acid reactions, formulas with non-ionized amino and carboxy groups are used.
1) reactions at the amino group. The amino group in amino acids exhibits the usual properties of amines: amines are bases and act as nucleophiles in reactions.
1. Reaction of amino acids as bases. When amino acids interact with acids, ammonium salts are formed:
glycine hydrochloride, glycine hydrochloride salt
2. Action of nitrous acid. When nitrous acid acts, hydroxy acids are formed and nitrogen and water are released:
This reaction is used for the quantitative determination of free amine groups in amino acids, as well as in proteins.
3. Formation of N - acyl derivatives, acylation reaction.
Amino acids react with anhydrides and acid halides, forming N - acyl derivatives of amino acids:
Benzyl ether sodium salt N carbobenzoxyglycine - chloroformic glycine
Acylation is one of the ways to protect the amino group. N-acyl derivatives are of great importance in the synthesis of peptides, since N-acyl derivatives are easily hydrolyzed to form a free amino group.
4. Formation of Schiff bases. When a-amino acids interact with aldehydes, substituted imines (Schiff bases) are formed through the stage of formation of carbinolamines:
alanine formaldehyde N-methylol derivative of alanine
5. Alkylation reaction. The amino group in the a-amino acid is alkylated to form N-alkyl derivatives:
The reaction with 2,4-dinitrofluorobenzene is of greatest importance. The resulting dinitrophenyl derivatives (DNP derivatives) are used in establishing the amino acid sequence of peptides and proteins. The interaction of a-amino acids with 2,4-dinitrofluorobenzene is an example of a nucleophilic substitution reaction in the benzene ring. Due to the presence of two strong electron-withdrawing groups in the benzene ring, the halogen becomes mobile and undergoes a substitution reaction:
 |
2.4 – dinitro -
fluorobenzene N - 2,4 - dinitrophenyl - a - amino acid
(DNPB) DNP - derivatives of a - amino acids
6.Reaction with phenyl isothiocyanate. This reaction is widely used in determining the structure of peptides. Phenyl isothiocyanate is a derivative of isothiocyanic acid H-N=C=S. The interaction of a-amino acids with phenyl isothiocyanate proceeds through the mechanism of a nucleophilic addition reaction. The resulting product then undergoes an intramolecular substitution reaction, leading to the formation of a cyclic substituted amide: phenylthiohydantoin.
Cyclic compounds are obtained in quantitative yield and are phenyl derivatives of thiohydantoin (PTH - derivatives) - amino acids. PTG derivatives differ in the structure of the R radical.
In addition to ordinary salts, a-amino acids can, under certain conditions, form intracomplex salts with heavy metal cations. All a-amino acids are characterized by beautifully crystallizing, intensely blue-colored intracomplex (chelate) copper salts):
Alanine ethyl ester
The formation of esters is one of the methods for protecting the carboxyl group in peptide synthesis.
3. Formation of acid halides. When acting on a-amino acids with a protected amino group with sulfur oxydichloride (thionyl chloride) or phosphorus oxide trichloride (phosphorus oxychloride), acid chlorides are formed:
The production of acid halides is one of the ways to activate the carboxyl group in peptide synthesis.
4.Obtaining a-amino acid anhydrides. Acid halides are very reactive, which reduces the selectivity of the reaction when used. Therefore, a more commonly used method for activating a carboxyl group in peptide synthesis is to convert it into an anhydride group. Anhydrides are less active than acid halides. When an a-amino acid having a protected amino group interacts with ethyl chloroformic acid (ethyl chloroformate), an anhydride bond is formed:
5. Decarboxylation. a - Amino acids that have two electron-withdrawing groups at the same carbon atom are easily decarboxylated. In laboratory conditions, this is carried out by heating amino acids with barium hydroxide. This reaction occurs in the body with the participation of decarboxylase enzymes with the formation of biogenic amines:
ninhydrin
Relation of amino acids to heat. When a-amino acids are heated, cyclic amides called diketopiperazines are formed:

Diketopiperazine
g - and d - Amino acids easily split off water and cyclize to form internal amides, lactams:
g - lactam (butyrolactam)
In cases where the amino and carboxyl groups are separated by five or more carbon atoms, when heated, polycondensation occurs with the formation of polymer polyamide chains with the elimination of a water molecule.





